Shifting machine learning for healthcare from development to deployment and from models to data
In the past decade, the application of machine learning (ML) to healthcare has helped drive the automation of physician tasks as well as enhancements in clinical capabilities and access to care. This progress has emphasized that, from model development to model deployment, data play central roles. In this Review, we provide a data-centric view of the innovations and challenges that are defining ML for healthcare. We discuss deep generative models and federated learning as strategies to augment datasets for improved model performance, as well as the use of the more recent transformer models for handling larger datasets and enhancing the modelling of clinical text. We also discuss data-focused problems in the deployment of ML, emphasizing the need to efficiently deliver data to ML models for timely clinical predictions and to account for natural data shifts that can deteriorate model performance.
Similar content being viewed by others
The future of digital health with federated learning
Article Open access 14 September 2020
Automated clinical coding: what, why, and where we are?
Article Open access 22 October 2022
Use of deep learning to develop continuous-risk models for adverse event prediction from electronic health records
Article 05 May 2021
Main
In the past decade, machine learning (ML) for healthcare has been marked by particularly rapid progress. Initial groundwork has been laid for many healthcare needs that promise to improve patient care, reduce healthcare workload, streamline healthcare processes and empower the individual 1 . In particular, ML for healthcare has been successful in the translation of computer vision through the development of image-based triage 2 and second readers 3 . There has also been rapid progress in the harnessing of electronic health records 4,5 (EHRs) to predict the risk and progression of many diseases 6,7 . A number of software platforms for ML are beginning to make their way into the clinic 8 . In 2018, iDX-DR, which detects diabetic retinopathy, was the first ML system for healthcare that the United States Food and Drug Administration approved for clinical use 8 . Babylon 9 , a chatbot triage system, has partnered with the United Kingdom’s National Healthcare system. Furthermore, Viz.ai 10,11 has rolled out their triage technology to more than 100 hospitals in the United States.
As ML systems begin to be deployed in clinical settings, the defining challenge of ML in healthcare has shifted from model development to model deployment. In bridging the gap between the two, another trend has emerged: the importance of data. We posit that large, well-designed, well-labelled, diverse and multi-institutional datasets drive performance in real-world settings far more than model optimization 12,13,14 , and that these datasets are critical for mitigating racial and socioeconomic biases 15 . We realize that such rich datasets are difficult to obtain, owing to clinical limitations of data availability, patient privacy and the heterogeneity of institutional data frameworks. Similarly, as ML healthcare systems are deployed, the greatest challenges in implementation arise from problems with the data: how to efficiently deliver data to the model to facilitate workflow integration and make timely clinical predictions? Furthermore, once implemented, how can model robustness be maintained in the face of the inevitability of natural changes in physician and patient behaviours? In fact, the shift from model development to deployment is also marked by a shift in focus: from models to data.
In this Review, we build on previous surveys 1,16,17 and take a data-centric approach to reviewing recent innovations in ML for healthcare. We first discuss deep generative models and federated learning as strategies for creating larger and enhanced datasets. We also examine the more recent transformer models for handling larger datasets. We end by highlighting the challenges of deployment, in particular, how to process and deliver usable raw data to models, and how data shifts can affect the performance of deployed models.
Deep generative models
Generative adversarial networks (GANs) are among the most exciting innovations in deep learning in the past decade. They offer the capability to create large amounts of synthetic yet realistic data. In healthcare, GANs have been used to augment datasets 18 , alleviate the problems of privacy-restricted 19 and unbalanced datasets 20 , and perform image-modality-to-image-modality translation 21 and image reconstruction 22 (Fig. 1). GANs aim to model and sample from the implicit density function of the input data 23 . They consist of two networks that are trained in an adversarial process under which one network, the ‘generator’, generates synthetic data while the other network, the ‘discriminator’, discriminates between real and synthetic data. The generative model aims to implicitly learn the data distribution from a set of samples to further generate new samples drawn from the learned distribution, while the discriminator pushes the generator network to sample from a distribution that more closely mirrors the true data distribution.
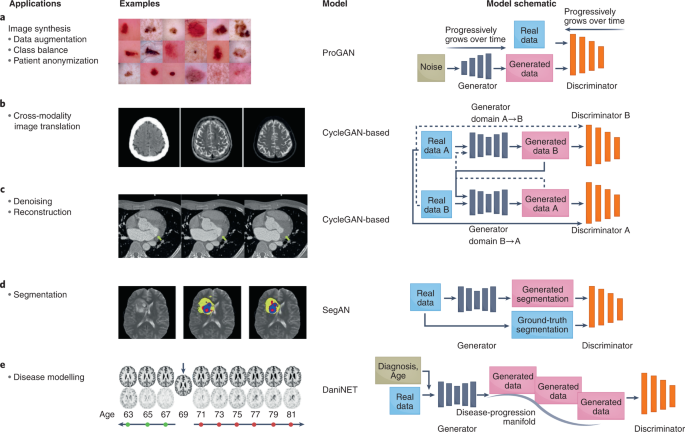
Over the years, a multitude of GANs have been developed to overcome the limitations of the original GAN (Table 1), and to optimize its performance and extend its functionalities. The original GAN 23 suffered from unstable training and low image diversity and quality 24 . In fact, training two adversarial models is, in practice, a delicate and often difficult task. The goal of training is to achieve a Nash equilibrium between the generator and the discriminator networks. However, simultaneously obtaining such an equilibrium for networks that are inherently adversarial is difficult and, if achieved, the equilibrium can be unstable (that is, it can be suddenly lost after model convergence). This has also led to sensitivity to hyperparameters (making the tuning of hyperparameters a precarious endeavour) and to mode collapse, which occurs when the generator produces a limited and repeated number of outputs. To remedy these limitations, changes have been made to GAN architectures and loss functions. In particular, the deep convolutional GAN (DCGAN 25 ), a popular GAN often used for medical-imaging tasks, aimed to combat instability by introducing key architecture-design decisions, including the replacement of fully connected layers with convolutional layers, and the introduction of batch normalization (to standardize the inputs to a layer when training deep neural networks) and ReLU (rectified linear unit) activation. The Laplacian pyramid of adversarial networks GAN (LAPGAN 26 ) and the progressively growing GAN (ProGAN 27 ) build on DCGAN to improve training stability and image quality. Both LAPGAN and ProGAN start with a small image, which promotes training stability, and progressively grow the image into a higher-resolution image.
Transfer learning for NLP
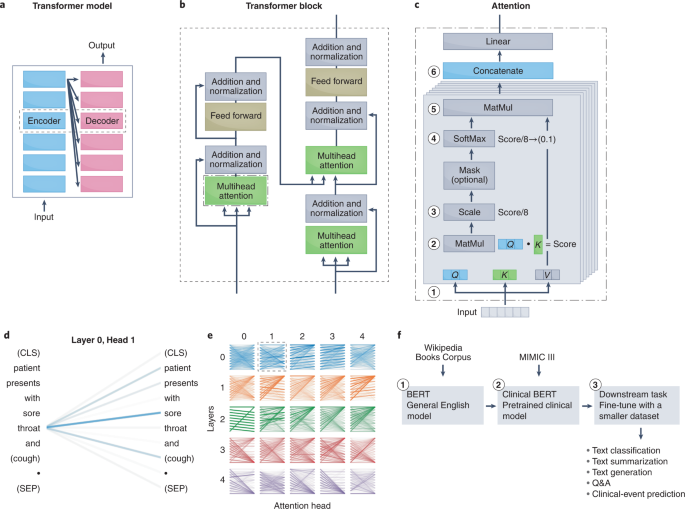
Simultaneous and subsequent work following the release of the transformer resolved another main problem in NLP: the formalization of the process of transfer learning. Transfer learning has been used most extensively in computer vision, owing to the success of the ImageNet challenge, which made pre-trained CNNs widely available 120 . Transfer learning has enabled the broader application of deep learning in healthcare 17 , as researchers can fine-tune a pre-trained CNN adept at image classification on a smaller clinical dataset to accomplish a wide spectrum of healthcare tasks 3,37,121,122 . Until recently, robust transfer learning for NLP models was not possible, which limited the use of NLP models in domain-specific applications. A series of recent milestones have enabled transfer learning for NLP. The identification of the ideal pre-training language task for deep-learning NLP models (for example, masked-language modelling, predicting missing words from surrounding context, next-sentence prediction or predicting whether two sentences follow one another) was solved by universal language model fine-tuning (ULM-FiT 123 ) and embeddings from language model (ELMo 124 ). The generative pre-trained transformer (GPT 125 ) from Open AI and the bidirectional encoder representations from transformers (BERT 126 ) from Google Brain then applied the methods formalized by ULM-FiT and ELMo to transformer models, delivering pre-trained models that achieved unprecedented capabilities on a series of NLP tasks.
Transformers for the understanding of clinical text
Following the success of transformers for NLP, their potential to handle domain-specific text, specifically clinical text, was quickly assessed. The performances of the transformer-based model BERT, the RNN-based model ELMo and traditional word-vector embeddings 127,128 at clinical-concept extraction (the identification of the medical problems, tests and treatments) from EHR data were evaluated 106 . BERT outperformed traditional word vectors by a substantial margin and was more computationally efficient than ELMo (it achieved higher performance with fewer training iterations) 129,130,131,132 . Pre-training on a dataset of 2 million clinical notes (the dataset multiparameter intelligence monitoring in intensive care 132 ; MIMIC-III) increased the performance of all NLP models. This suggests that contextual embeddings encode valuable semantic information not accounted for in traditional word representations 106 . However, the performance of MIMIC-III BERT began to decline after achieving its optimal model; this is perhaps indicative of the model losing information learned from the large open corpus and converging to a model similar to the one initialized from scratch 106 . Hence, there may be a fine balance between learning from a large open-domain corpus and a domain-specific clinical corpus. This may be a critical consideration when applying pre-trained models to healthcare tasks.
To facilitate the further application of clinically pre-trained BERT 129 to downstream clinical tasks, a BERT pre-trained on large clinical datasets was publicly released. Because transformers and deep NLP models are resource-intensive to train (training the BERT model can cost US$50,000–200,000 133 ; and pre-training BERT on clinical datasets required 18 d of continuous training, an endeavour that may be out of the reach of many institutions), openly releasing pre-trained clinical models can facilitate widespread advancements of NLP tasks in healthcare. Other large and publicly available clinically pre-trained models (Table 3) are ClinicalBERT 130 , BioBERT 134 and SciBERT 135 .
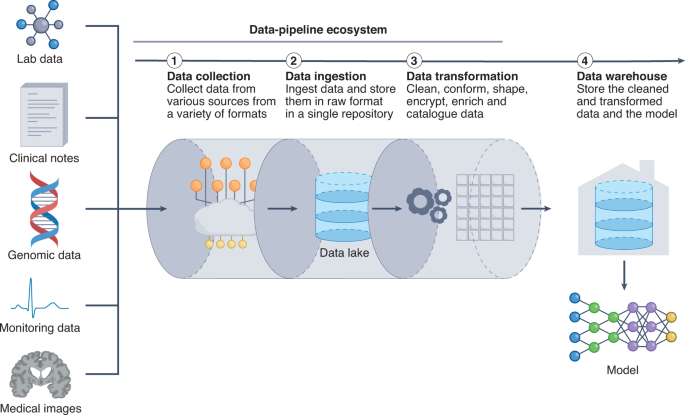
The fundamental challenge of creating an adept data pipeline arises from the need to anticipate the heterogeneity of the data. ML models often require a set of specific clinical inputs (for example, blood pressure and heart rate), which are extracted from a suite of dynamically changing health data. However, it is difficult to extract the relevant data inputs. Clinical data vary in volume and velocity (the rate that data are generated), thus prompting the question of how frequently data should be collected. Furthermore, clinical data can vary in veracity (data quality), thus requiring different pre-processing steps. Moreover, the majority of clinical data exist in an unstructured format that is further complicated by the availability of hundreds of EHR products, each with its own clinical terminology, technical specifications and capabilities 156 . Therefore, how to precisely extract data from a spectrum of unstructured EHR frameworks becomes critical.
Data heterogeneity must be carefully accounted for when designing the data pipeline, as it can influence throughput, latency and other performance factors. The data pipeline starts with the process of data ingestion (by which raw clinical data are moved from the data source and into the pipeline), a primary bottleneck in the throughput of the data through the pipeline. In particular, handling peaks of data generation may require the design and implementation of scalable ways to support a variable number of connected objects 157 . Such data-elasticity issues can take advantage of software frameworks that scale up or down in real time to more effectively use computer resources in cloud data centres 158 .
After the data enters the pipeline, the data-preparation stage involves the cleansing, denoising, standardization and shaping of the data into structured data that are ready for consumption by the ML system. In studies that developed data pipelines to handle healthcare data 156,159,160 , the data-preparation stage was found to regulate the latency of the data pipeline, as latency depended on the efficiency of the data queue, the streaming of the data and the database for storing the computation results.
A final consideration is how data should move throughout the data pipeline; specifically, whether data should move in discrete batches or in continuous streams. Batch processing involves collecting and moving source data periodically, whereas stream processing involves sourcing, moving and processing data as soon as they are created. Batch processing has the advantages of being high-throughput, comprehensive and economical (and hence may be advantageous for scalability), whereas stream processing occurs in real time (and thus may be required for time-sensitive predictions). Many healthcare systems use a combination of batch processing and stream processing 160 .
Established data pipelines are being harnessed to support real-time healthcare modelling. In particular, Columbia University Medical Center, in collaboration with IBM, is streaming physiological data from patients with brain injuries to predict adverse neurological complications up to 48 h before existing methods can 161 . Similarly, Yale School of Medicine has used a data pipeline to support real-time data acquisition for predicting the number of beds available, handling care for inpatients and patients in the intensive care unit (such as managing ventilator capacity) and tracking the number of healthcare providers exposed to COVID-19 161 . However, optimizing the components of the data pipeline, particularly for numerous concurrent ML healthcare systems, remains a challenging task.
Deployment in the face of data shifts
A main obstacle in deploying ML systems for healthcare has been maintaining model robustness when faced with data shifts 162 . Data shifts occur when differences or changes in healthcare practices or in patient behaviour cause the deployment data to differ substantially from the training data, resulting in the distribution of the deployment data diverging from the distribution of the training data. This can lead to a decline in model performance. Also, failure to correct for data shifts can lead to the perpetuation of algorithmic biases, missing critical diagnoses 163 and unnecessary clinical interventions 164 .
In healthcare, data shifts are common occurrences and exist primarily along the axes of institutional differences (such as local clinical practices, or different instruments and data-collection workflows), epidemiological shifts, temporal shifts (for example, changes in physician and patient behaviours over time) and differences in patient demographics (such as race, gender and age). A recent case study 165 characterizing data shifts caused by institutional differences reported that pneumothorax classifiers trained on individual institutional datasets declined in performance when evaluated on data from external institutions. Similar phenomena have been observed in a number of studies 41,163,166 . Institutional differences are among the most patent causes of data shifts because they frequently harbour underlying differences in patient demographics, disease incidence and data-collection workflows. For example, in an analysis of chest-X-ray classifiers and their potential to generalize to other institutions, it was found that one institution collected chest X-rays using portable radiographs, whereas another used stationary radiographs 41 . This led to differences in disease prevalence (33% vs 2% for pneumonia) and patient demographics (average age of 63 vs 45), as portable radiographs were primarily used for inpatients who were too sick to be transported, whereas stationary radiographs were used primarily in outpatient settings. Similarly, another study found that different image-acquisition and image-processing techniques caused the deterioration of the performance of breast-mammography classifiers to random performance (areas under the receiver operating characteristic curve of 0.4–0.6) when evaluated on datasets from four external institutions and countries 163 . However, it is important to note that the models evaluated were trained on data collected during the 1990s and were externally tested on datasets created in 2014–2017. The decline in performance owing to temporal shifts is particularly relevant; if deployed today, models that have been trained on older datasets would be making inferences on newly generated data.
Studies that have characterized temporal shifts have provided insights into the conditions under which deployed ML models should be re-evaluated. An evaluation of models that used data collected over a period of 9 years found that model performance deteriorated substantially, drifting towards overprediction as early as one year after model development 167 . For the MIMIC-III dataset 132 (commonly used for the development of models to predict clinical outcomes), an assessment of the effects of temporal shifts on model performance over time showed that, whereas all models experienced a moderate decline over time, the most significant drop in performance occurred owing to a shift in clinical practice, when EHRs transitioned systems 164 (from CareVue to MetaVision). A modern-day analogy would be how ML systems for COVID-19 (ref. 168 ) that were trained on data 169 acquired during the early phase of the pandemic and before the availability of COVID-19 vaccines would perform when deployed in the face of shifts in disease incidence and presentation.
Data shifts and model deterioration can also occur when models are deployed on patients with gender, racial or socioeconomic backgrounds that are different from those of the patient population that the model was trained on. In fact, it has been shown that ML models can be biased against individuals of certain races 170 or genders 42 , or particular religious 171 or socioeconomic 15 backgrounds. For example, a large-scale algorithm used in many health institutions to identify patients for complex health needs underpredicted the health needs of African American patients and failed to triage them for necessary care 172 . Using non-representative or non-inclusive training datasets can constitute an additional source of gender, racial or socioeconomic biases. Popular chest-X-ray datasets used to train classifiers have been shown to be heavily unbalanced 15 : 67.6% of the patients in these datasets are Caucasian and only 8.98% are under Medicare insurance. Unsurprisingly, the performance of models trained with these datasets deteriorates for non-Caucasian subgroups, and especially for Medicare patients 15 . Similarly, skin-lesion classifiers that were trained primarily on images of one skin tone decrease in performance when evaluated on images of different skin tones 173 ; in this case, the drop in performance could be attributed to variations in disease presentation that are not captured when certain patient populations are not adequately represented in the training dataset 174 .
These findings exemplify two underlying limitations of ML models: the models can propagate existing healthcare biases on a large scale, and insufficient diversity in the training datasets can lead to an inadequate generalization of model outputs to different patient populations. Training models on multi-institutional datasets can be most effective at combating model deterioration 15 , and directly combating existing biases in the training data can also mitigate their impact 171 . There are also solutions for addressing data shifts that involve proactively addressing them during model development 175,176,177,178 or retroactively by surveilling for data shifts during model deployment 179 . A proactive attitude towards recognizing and addressing potential biases and data shifts will remain imperative.
Outlook
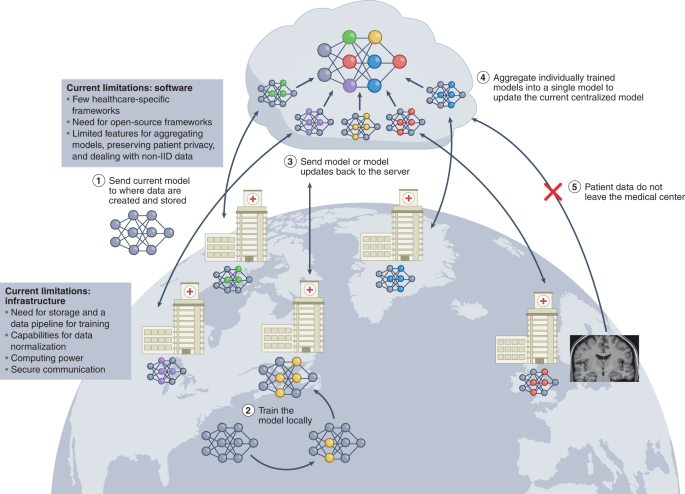
Substantial progress in the past decade has laid a foundation of knowledge for the application of ML to healthcare. In pursuing the deployment of ML models, it is clear that success is dictated by how data are collected, organized, protected, moved and audited. In this Review, we have highlighted methods that can address these challenges. The emphasis will eventually shift to how to build the tools, infrastructure and regulations needed to efficiently deploy innovations in ML in clinical settings. A central challenge will be the implementation and translation of these advances into healthcare in the face of their current limitations: for instance, GANs applied to medical images are currently limited by image resolution and image diversity, and can be challenging to train and scale; federated learning promises to alleviate problems associated with small single-institution datasets, yet it requires robust frameworks and infrastructure; and large language models trained on large public datasets can subsume racial and ethnic biases 171 .
Another central consideration is how to handle the regulatory assessment of ML models for healthcare applications. Current regulation and approval processes are being adapted to meet the emerging needs; in particular, initiatives are attempting to address data shifts and patient representation in the training datasets 165,180,181 . However, GANs, federated learning and transformer models add complexities to the regulatory process. Few healthcare-specific benchmarking datasets exist to evaluate the performance of these ML systems during clinical deployment. Moreover, the assessment of the performance of GANs is hampered by the lack of efficient and robust metrics to evaluate, compare and control the quality of synthetic data.
Notwithstanding the challenges, the fact that analogous ML technologies are being used daily by millions of individuals in other domains, most prominently in smartphones 100 , search engines 182 and self-driving vehicles 68 , suggests that the challenges of deployment and regulation of ML for healthcare can also be addressed.
References
- Topol, E. J. High-performance medicine: the convergence of human and artificial intelligence. Nat. Med.25, 44–56 (2019). ArticleCASGoogle Scholar
- Gulshan, V. et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA316, 2402–2410 (2016). ArticleGoogle Scholar
- Esteva, A. et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature542, 115–118 (2017). ArticleCASGoogle Scholar
- Rajkomar, A. et al. Scalable and accurate deep learning with electronic health records. npj Digit. Med.1, 18 (2018). ArticleGoogle Scholar
- Rajkomar, A. et al. Automatically charting symptoms from patient-physician conversations using machine learning. JAMA Intern. Med.179, 836–838 (2019). ArticleGoogle Scholar
- Henry, K. E., Hager, D. N., Pronovost, P. J. & Saria, S. A targeted real-time early warning score (TREWScore) for septic shock. Sci. Transl. Med.7, 299ra122 (2015). ArticleGoogle Scholar
- Komorowski, M., Celi, L. A., Badawi, O., Gordon, A. C. & Faisal, A. A. The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat. Med.24, 1716–1720 (2018). ArticleCASGoogle Scholar
- Abràmoff, M. D., Lavin, P. T., Birch, M., Shah, N. & Folk, J. C. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. npj Digit. Med.1, 39 (2018). ArticleGoogle Scholar
- Iacobucci, G. Babylon Health holds talks with ‘significant’ number of NHS trusts. Brit. Med. J.368, m266 (2020). ArticleGoogle Scholar
- Hale, C. Medtronic to distribute Viz.ai’s stroke-spotting AI imaging software. Fierce Biotech (23 July 2019); https://www.fiercebiotech.com/medtech/medtronic-to-distribute-viz-ai-s-stroke-spotting-ai-imaging-software
- Hassan, A. E. et al. Early experience utilizing artificial intelligence shows significant reduction in transfer times and length of stay in a hub and spoke model. Interv. Neuroradiol.26, 615–622 (2020). ArticleGoogle Scholar
- Ting, D. S. W. et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA318, 2211–2223 (2017). ArticleGoogle Scholar
- McKinney, S. M. et al. International evaluation of an AI system for breast cancer screening. Nature577, 89–94 (2020). ArticleCASGoogle Scholar
- Liu, Y. et al. A deep learning system for differential diagnosis of skin diseases. Nat. Med.26, 900–908 (2020). ArticleCASGoogle Scholar
- Seyyed-Kalantari, L., Liu, G., McDermott, M., Chen, I. Y. & Ghassemi, M. CheXclusion: fairness gaps in deep chest X-ray classifiers. Pac. Symp. Biocomput.26, 232–243 (2021). Google Scholar
- Yu, K.-H., Beam, A. L. & Kohane, I. S. Artificial intelligence in healthcare. Nat. Biomed. Eng.2, 719–731 (2018). ArticleGoogle Scholar
- Esteva, A. et al. A guide to deep learning in healthcare. Nat. Med.25, 24–29 (2019). ArticleCASGoogle Scholar
- Frid-Adar, M. et al. GAN-based synthetic medical image augmentation for increased CNN performance in liver lesion classification. Neurocomputing321, 321–331 (2018). ArticleGoogle Scholar
- Shin, H.-C. et al. Medical image synthesis for data augmentation and anonymization using generative adversarial networks. In Simulation and Synthesis in Medical Imaging SASHIMI 2018 (eds Gooya, A., Goksel, O., Oguz, I. & Burgos, N.) 1–11 (Springer Cham, 2018).
- Salehinejad, H., Valaee, S., Dowdell, T., Colak, E. & Barfett, J. Generalization of deep neural networks for chest pathology classification in X-rays using generative adversarial networks. In IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP) 990–994 (ieeexplore.ieee.org, 2018).
- Zhang, Z., Yang, L. & Zheng, Y. Translating and segmenting multimodal medical volumes with cycle-and shape-consistency generative adversarial network. In Proceedings of IEEE/CVF Conference on Computer Vision and Pattern Recognition 9242–9251 (IEEE, 2018).
- Xu, F., Zhang, J., Shi, Y., Kang, K. & Yang, S. A fast low-rank matrix factorization method for dynamic magnetic resonance imaging restoration. In 5th International Conference on Big Data Computing and Communications (BIGCOM) 38–42 (2019).
- Goodfellow, I. J. et al. Generative adversarial networks. In Advances in Neural Information Processing Systems 27 (eds .Ghahramani, Z., Welling, M., Cortes, C., Lawrence, N. & Weinbergerm, K.Q.) Paper 1384 (Curran, 2014).
- Wang, Z., She, Q. & Ward, T. E. Generative adversarial networks in computer vision: a survey and taxonomy. ACM Comput. Surv.54, 1–38 (2021). ArticleGoogle Scholar
- Radford, A., Metz, L. & Chintala, S. Unsupervised representation learning with deep convolutional generative adversarial networks. Preprint at https://arxiv.org/abs/1511.06434v2 (2016).
- Denton, E. L., Chintala, S. & Fergus, R. Deep generative image models using a Laplacian pyramid of adversarial networks. In Advances in Neural Information Processing Systems 28 (eds Cortes, C., Lawrence, N., Lee, D., Sugiyama, M. & Garnett, R.) Paper 903 (Curran, 2015).
- Karras, T., Aila, T., Laine, S. & Lehtinen, J. Progressive growing of GANs for improved quality, stability, and variation. In International Conference on Learning Representations 2018 Paper 447 (ICLR, 2018).
- Mirza, M. & Osindero, S. Conditional generative adversarial nets. Preprint at https://arxiv.org/abs/1411.1784v1 (2014).
- Odena, A., Olah, C. & Shlens, J. Conditional image synthesis with auxiliary classifier GANs. In Proceedings of the 34th International Conference on Machine Learning (eds. Precup, D. & Teh, Y. W.) 2642–2651 (PMLR, 2017).
- Isola, P., Zhu, J.-Y., Zhou, T. & Efros, A. A. Image-to-image translation with conditional adversarial networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition 5967–5976 (2018).
- Zhang, H., Goodfellow, I., Metaxas, D. & Odena, A. Self-attention generative adversarial networks. In Proceedings of the 36th International Conference on Machine Learning (eds. Chaudhuri, K. & Salakhutdinov, R.) 7354–7363 (PMLR, 2019).
- Wu, Y., Ma, Y., Liu, J., Du, J. & Xing, L. Self-attention convolutional neural network for improved MR image reconstruction. Inf. Sci.490, 317–328 (2019). ArticleGoogle Scholar
- Brock, A., Donahue, J. & Simonyan, K. Large scale GAN training for high fidelity natural image synthesis. In International Conference on Learning Representations Paper 564 (ICLR, 2019).
- Arjovsky, M., Chintala, S. & Bottou, L. Wasserstein generative adversarial networks. In Proceedings of the 34th International Conference on Machine Learning (eds. Precup, D. & Teh, Y. W.) 214–223 (PMLR, 2017).
- Gulrajani, I., Ahmed, F., Arjovsky, M., Dumoulin, V. & Courville, A. C. Improved training of Wasserstein GANs. In Advances in Neural Information Processing Systems 30 (eds. Guyon, I. et al.) Paper 2945 (Curran, 2017).
- Hindupur, A. The-gan-zoo. https://github.com/hindupuravinash/the-gan-zoo (2018).
- Rajpurkar, P. et al. Deep learning for chest radiograph diagnosis: a retrospective comparison of the CheXNeXt algorithm to practicing radiologists. PLoS Med.15, e1002686 (2018). ArticleGoogle Scholar
- Ouyang, D. et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature580, 252–256 (2020). ArticleCASGoogle Scholar
- Xue, Y., Xu, T., Zhang, H., Long, L. R. & Huang, X. SegAN: adversarial network with multi-scale L1 loss for medical image segmentation. Neuroinformatics16, 383–392 (2018). ArticleGoogle Scholar
- Haque, A., Milstein, A. & Fei-Fei, L. Illuminating the dark spaces of healthcare with ambient intelligence. Nature585, 193–202 (2020). ArticleCASGoogle Scholar
- Zech, J. R. et al. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: a cross-sectional study. PLoS Med.15, e1002683 (2018). ArticleGoogle Scholar
- Zou, J. & Schiebinger, L. AI can be sexist and racist — it’s time to make it fair. Nature559, 324–326 (2018). ArticleCASGoogle Scholar
- Perez, L. & Wang, J. The effectiveness of data augmentation in image classification using deep learning. Preprint at https://arxiv.org/abs/1712.04621v1 (2017).
- Madani, A., Moradi, M., Karargyris, A. & Syeda-Mahmood, T. Semi-supervised learning with generative adversarial networks for chest X-ray classification with ability of data domain adaptation. In IEEE 15th International Symposium on Biomedical Imaging (ISBI) 1038–1042 (IEEE, 2018).
- He, J. et al. The practical implementation of artificial intelligence technologies in medicine. Nat. Med.25, 30–36 (2019). ArticleCASGoogle Scholar
- Kelly, C. J., Karthikesalingam, A., Suleyman, M., Corrado, G. & King, D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med.17, 195 (2019). ArticleGoogle Scholar
- Rocher, L., Hendrickx, J. M. & de Montjoye, Y.-A. Estimating the success of re-identifications in incomplete datasets using generative models. Nat. Commun.10, 3069 (2019). ArticleGoogle Scholar
- Schwarz, C. G. et al. Identification of anonymous MRI research participants with face-recognition software. N. Engl. J. Med.381, 1684–1686 (2019). ArticleGoogle Scholar
- Chartsias, A., Joyce, T., Dharmakumar, R. & Tsaftaris, S. A. Adversarial image synthesis for unpaired multi-modal cardiac data. in Simulation and Synthesis in Medical Imaging (eds. Tsaftaris, S. A., Gooya, A., Frangi, A. F. & Prince, J. L.) 3–13 (Springer International Publishing, 2017).
- Emami, H., Dong, M., Nejad-Davarani, S. P. & Glide-Hurst, C. K. Generating synthetic CTs from magnetic resonance images using generative adversarial networks. Med. Phys. https://doi.org/10.1002/mp.13047 (2018).
- Jin, C.-B. et al. Deep CT to MR synthesis using paired and unpaired data. Sensors19, 2361 (2019). ArticleGoogle Scholar
- Bi, L., Kim, J., Kumar, A., Feng, D. & Fulham, M. In Molecular Imaging, Reconstruction and Analysis of Moving Body Organs, and Stroke Imaging and Treatment (eds. Cardoso, M. J. et al.) 43–51 (Springer International Publishing, 2017).
- Ben-Cohen, A. et al. Cross-modality synthesis from CT to PET using FCN and GAN networks for improved automated lesion detection. Eng. Appl. Artif. Intell.78, 186–194 (2019).
- Armanious, K. et al. MedGAN: medical image translation using GANs. Comput. Med. Imaging Graph.79, 101684 (2020). ArticleGoogle Scholar
- Choi, H. & Lee, D. S. Alzheimer’s Disease Neuroimaging Initiative. Generation of structural MR images from amyloid PET: application to MR-less quantification. J. Nucl. Med.59, 1111–1117 (2018). ArticleCASGoogle Scholar
- Wei, W. et al. Learning myelin content in multiple sclerosis from multimodal MRI through adversarial training. In Medical Image Computing and Computer Assisted Intervention — MICCAI 2018 (eds. Frangi, A. F., Schnabel, J. A., Davatzikos, C., Alberola-López, C. & Fichtinger, G.) 514–522 (Springer Cham, 2018).
- Pan, Y. et al. Synthesizing missing PET from MRI with cycle-consistent generative adversarial networks for Alzheimer’s disease diagnosis. In Medical Image Computing and Computer Assisted Intervention— MICCAI 2018 (eds. Frangi, A. F., Schnabel, J. A., Davatzikos, C., Alberola-López, C. & Fichtinger, G.) 455–463 (Springer Cham, 2018).
- Welander, P., Karlsson, S. & Eklund, A. Generative adversarial networks for image-to-image translation on multi-contrast MR images - a comparison of CycleGAN and UNIT. Preprint at https://arxiv.org/abs/1806.07777v1 (2018).
- Dar, S. U. H. et al. Image synthesis in multi-contrast MRI with conditional generative adversarial networks. IEEE Trans. Med. Imaging38, 2375–2388 (2019).
- Zhu, J.-Y., Park, T., Isola, P. & Efros, A. A. Unpaired image-to-image translation using cycle-consistent adversarial networks. In 2017 IEEE International Conference on Computer Vision (ICCV) (IEEE, 2017); https://doi.org/10.1109/iccv.2017.244
- Maspero, M. et al. Dose evaluation of fast synthetic-CT generation using a generative adversarial network for general pelvis MR-only radiotherapy. Phys. Med. Biol.63, 185001 (2018). ArticleGoogle Scholar
- Olut, S., Sahin, Y.H., Demir, U., Unal, G. Generative adversarial training for MRA image synthesis using multi-contrast MRI. In PRedictive Intelligence in MEdicine. PRIME 2018. Lecture Notes in Computer Science (eds Rekik, I., Unal, G., Adeli, E. & Park, S.) (Springer Cham, 2018); https://doi.org/10.1007/978-3-030-00320-3_18
- Chen, R. J., Lu, M. Y., Chen, T. Y., Williamson, D. F. K. & Mahmood, F. Synthetic data in machine learning for medicine and healthcare. Nat. Biomed. Eng.5, 493–497 (2021). ArticleGoogle Scholar
- Kanakasabapathy, M. K. et al. Adaptive adversarial neural networks for the analysis of lossy and domain-shifted datasets of medical images. Nat. Biomed. Eng.5, 571–585 (2021). ArticleGoogle Scholar
- Bowles, C., Gunn, R., Hammers, A. & Rueckert, D. Modelling the progression of Alzheimer’s disease in MRI using generative adversarial networks. In Medical Imaging 2018: Image Processing (eds. Angelini, E. D. & Landman, B. A.) 397– 407 (International Society for Optics and Photonics, 2018).
- Ravi, D., Alexander, D.C., Oxtoby, N.P. & Alzheimer’s Disease Neuroimaging Initiative. Degenerative adversarial neuroImage nets: generating images that mimic disease progression. In Medical Image Computing and Computer Assisted Intervention — MICCAI 2019. Lecture Notes in Computer Science. (eds Shen, D. et al) 164–172 (Springer, 2019).
- Borji, A. Pros and cons of GAN evaluation measures. Comput. Vis. Image Underst.179, 41–65 (2019). ArticleGoogle Scholar
- Vincent, J. Nvidia uses AI to make it snow on streets that are always sunny. The Vergehttps://www.theverge.com/2017/12/5/16737260/ai-image-translation-nvidia-data-self-driving-cars (2017).
- Kairouz, P. et al. Advances and open problems in federated learning. Found. Trends Mach. Learn.https://doi.org/10.1561/2200000083 (2021)
- McMahan, B., Moore, E., Ramage, D., Hampson, S. & Aguera y Arcas, B. Communication-efficient learning of deep networks from decentralized data. In Proceedings of the 20th International Conference on Artificial Intelligence and Statistics (eds. Singh, A. & Zhu, J.) 1273–1282 (ML Research Press, 2017).
- Li, X. et al. Multi-site fMRI analysis using privacy-preserving federated learning and domain adaptation: ABIDE results. Med. Image Anal.65, 101765 (2020). ArticleGoogle Scholar
- Brisimi, T. S. et al. Federated learning of predictive models from federated Electronic Health Records. Int. J. Med. Inform.112, 59–67 (2018). ArticleGoogle Scholar
- Lee, J. et al. Privacy-preserving patient similarity learning in a federated environment: development and analysis. JMIR Med. Inform.6, e20 (2018). ArticleGoogle Scholar
- Dou, Q. et al. Federated deep learning for detecting COVID-19 lung abnormalities in CT: a privacy-preserving multinational validation study. npj Digit. Med.4, 60 (2021). ArticleGoogle Scholar
- Silva, S. et al. Federated learning in distributed medical databases: meta-analysis of large-scale subcortical brain data. In 2019 IEEE 16th International Symposium on Biomedical Imaging ISBI 2019 18822077 (IEEE, 2019).
- Sheller, M. J., Reina, G. A., Edwards, B., Martin, J. & Bakas, S. Multi-institutional deep learning modeling without sharing patient data: a feasibility study on brain tumor segmentation. Brainlesion11383, 92–104 (2019). Google Scholar
- Sheller, M. J. et al. Federated learning in medicine: facilitating multi-institutional collaborations without sharing patient data. Sci. Rep.10, 12598 (2020). ArticleGoogle Scholar
- Sarma, K. V. et al. Federated learning improves site performance in multicenter deep learning without data sharing. J. Am. Med. Inform. Assoc.28, 1259–1264 (2021). ArticleGoogle Scholar
- Li, W. et al. Privacy-preserving federated brain tumour segmentation. In Machine Learning in Medical Imaging (eds. Suk, H.-I., Liu, M., Yan, P. & Lian, C.) 133–141 (Springer International Publishing, 2019).
- Shokri, R., Stronati, M., Song, C. & Shmatikov, V. Membership inference attacks against machine learning models. In IEEE Symposium on Security and Privacy SP 2017 3–18 (IEEE, 2017).
- Fredrikson, M., Jha, S. & Ristenpart, T. Model inversion attacks that exploit confidence information and basic countermeasures. In Proceedings of the 22nd ACM SIGSAC Conference on Computer and Communications Security 1322–1333 (Association for Computing Machinery, 2015).
- Zhang, C., Bengio, S., Hardt, M., Recht, B. & Vinyals, O. Understanding deep learning (still) requires rethinking generalization. Commun. ACM64, 107–115 (2021). ArticleGoogle Scholar
- Zhu, L., Liu, Z. & Han, S. Deep leakage from gradients. In Advances in Neural Information Processing Systems 32 (eds Wallach, H. et al.) Paper 8389 (Curran, 2019)
- Abadi, M. et al. Deep learning with differential privacy. In Proceedings of the 2016 ACM SIGSAC Conference on Computer and Communications Security 308–318 (Association for Computing Machinery, 2016).
- Brendan McMahan, H. et al. A general approach to adding differential privacy to iterative training procedures. Preprint at https://arxiv.org/abs/1812.06210v2 (2018).
- McMahan, H. B., Ramage, D., Talwar, K. & Zhang, L. Learning differentially private recurrent language models. In ICLR 2018 Sixth International Conference on Learning Representations Paper 504 (ICLR, 2018).
- Shokri, R. & Shmatikov, V. Privacy-preserving deep learning. In Proceedings of the 22nd ACM SIGSAC Conference on Computer and Communications Security 1310–1321 (Association for Computing Machinery, 2015).
- Lyu, M., Su, D. & Li, N. Understanding the sparse vector technique for differential privacy. Proc. VLDB Endow.10, 637–648 (2017). ArticleGoogle Scholar
- Hitaj, B., Ateniese, G. & Perez-Cruz, F. Deep models under the GAN: information leakage from collaborative deep learning. In Proceedings of the 2017 ACM SIGSAC Conference on Computer and Communications Security 603–618 (Association for Computing Machinery, 2017).
- Li, X., Huang, K., Yang, W., Wang, S. & Zhang, Z. On the convergence of FedAvg on Non-IID Data. In ICLR 2020 Eighth International Conference on Learning Representations Paper 261 (2020).
- Smith, V., Chiang, C.-K., Sanjabi, M. & Talwalkar, A. S. Federated multi-task learning. In Advances in Neural Information Processing Systems 30 (eds Guyon, I. et al.) Paper 2307 (NeuIPS, 2017).
- Xu, J. et al. Federated learning for healthcare informatics. J. Healthc. Inform. Res.5, 1–19 (2021). ArticleGoogle Scholar
- Huang, L. et al. LoAdaBoost: loss-based AdaBoost federated machine learning with reduced computational complexity on IID and non-IID intensive care data. PLoS ONE15, e0230706 (2020). ArticleCASGoogle Scholar
- Zhao, Y. et al. Federated learning with non-IID data. Preprint at https://arxiv.org/abs/1806.00582v1 (2018).
- Torres-Soto, J. & Ashley, E. A. Multi-task deep learning for cardiac rhythm detection in wearable devices. npj Digit. Med.3, 116 (2020). ArticleGoogle Scholar
- Turakhia, M. P. et al. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: The Apple Heart Study. Am. Heart J.207, 66–75 (2019). ArticleGoogle Scholar
- Synced. Apple reveals design of its on-device ML system for federated evaluation and tuning SyncedReviewhttps://syncedreview.com/2021/02/19/apple-reveals-design-of-its-on-device-ml-system-for-federated-evaluation-and-tuning (2021).
- McMahan, B. & Ramage, D. Federated learning: collaborative machine learning without centralized training data Google AI Bloghttps://ai.googleblog.com/2017/04/federated-learning-collaborative.html (2017).
- Chen, Y., Qin, X., Wang, J., Yu, C. & Gao, W. FedHealth: a federated transfer learning framework for wearable healthcare. IEEE Intell. Syst.35, 83–93 (2020). ArticleGoogle Scholar
- Ramage, D. & Mazzocchi, S. Federated analytics: collaborative data science without data collection Google AI Bloghttps://ai.googleblog.com/2020/05/federated-analytics-collaborative-data.html (2020).
- Augenstein, S. et al. Generative models for effective ML on private, decentralized datasets. In ICLR 2020 Eighth International Conference on Learning Representations Paper 1448 (ICLR, 2020).
- Pati, S. et al. The federated tumor segmentation (FeTS) challenge. Preprint at https://arxiv.org/abs/2105.05874v2 (2021).
- Flores, M. Medical institutions collaborate to improve mammogram assessment AI with Nvidia Clara federated learning The AI Podcasthttps://blogs.nvidia.com/blog/2020/04/15/federated-learning-mammogram-assessment/ (2020).
- Kannan, A., Chen, K., Jaunzeikare, D. & Rajkomar, A. Semi-supervised learning for information extraction from dialogue. In Proc. Interspeech 2018 2077–2081 (ISCA, 2018); https://doi.org/10.21437/interspeech.2018-1318
- Chiu, C.-C. et al. Speech recognition for medical conversations. Preprint at https://arxiv.org/abs/1711.07274v2; https://doi.org/10.1093/jamia/ocx073 (2017).
- Si, Y., Wang, J., Xu, H. & Roberts, K. Enhancing clinical concept extraction with contextual embeddings. J. Am. Med. Inform. Assoc.26, 1297–1304 (2019). ArticleGoogle Scholar
- Shin, H.-C. et al. Learning to read chest X-rays: recurrent neural cascade model for automated image annotation. In 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR) (IEEE, 2016); https://doi.org/10.1109/cvpr.2016.274
- Wang, X., Peng, Y., Lu, L., Lu, Z. & Summers, R. M. TieNet: text-image embedding network for common thorax disease classification and reporting in chest X-rays. In IEEE/CVF Conference on Computer Vision and Pattern Recognition 2018 (IEEE, 2018); https://doi.org/10.1109/cvpr.2018.00943
- Hochreiter, S. & Schmidhuber, J. Long short-term memory. Neural Comput.9, 1735–1780 (1997). ArticleCASGoogle Scholar
- Cho, K. et al. Learning phrase representations using RNN encoder-decoder for statistical machine translation. In Proceedings of the 2014 Conference on Empirical Methods in Natural Language Processing (EMNLP) (eds Moschitti, A., Pang, B. & Daelemans, W.) 1724–1734 (Association for Computational Linguistics, 2014).
- Lipton, Z. C., Kale, D. C., Elkan, C. & Wetzel, R. Learning to diagnose with LSTM recurrent neural networks. Preprint at https://arxiv.org/abs/1511.03677v7 (2015).
- Choi, E., Bahadori, M. T., Schuetz, A., Stewart, W. F. & Sun, J. Doctor AI: predicting clinical events via recurrent neural networks. JMLR Workshop Conf. Proc.56, 301–318 (2016). Google Scholar
- Zhu, Paschalidis & Tahmasebi. Clinical concept extraction with contextual word embedding. Preprint at https://doi.org/10.48550/arXiv.1810.10566 (2018).
- Cho, K., van Merriënboer, B., Bahdanau, D. & Bengio, Y. On the properties of neural machine translation: encoder–decoder approaches. In Proceedings of SSST-8, Eighth Workshop on Syntax, Semantics and Structure in Statistical Translation (eds Wu, D., Carpuat, M., Carreras, X. & Vecchi, E. M.) 103–111 (Association for Computational Linguistics, 2014).
- Gehring, J., Auli, M., Grangier, D., Yarats, D. & Dauphin, Y. N. Convolutional sequence to sequence learning. In Proceedings of the 34th International Conference on Machine Learning (eds Precup, D. & Teh, Y. W.) 1243–1252 (PMLR, 2017).
- Vaswani, A. et al. Attention is all you need. In Advances in Neural Information Processing Systems 30 (eds Guyon, I. et al.) Paper 3058 (Curran, 2017).
- Bahdanau, D., Cho, K. H. & Bengio, Y. Neural machine translation by jointly learning to align and translate. In 3rd International Conference on Learning Representations ICLR 2015 (ICLR, 2015).
- Luong, T., Pham, H. & Manning, C. D. Effective approaches to attention-based neural machine translation. In Proceedings of the 2015 Conference on Empirical Methods in Natural Language Processing (eds Màrquez, L., Callison-Burch, C. & Su, J.) 1412–1421 (Association for Computational Linguistics, 2015); https://doi.org/10.18653/v1/d15-1166
- Sutskever, I., Vinyals, O. & Le, Q. V. Sequence to sequence learning with neural networks. In Advances in Neural Information Processing Systems 27 (eds Ghahramani, Z., Welling, M., Cortes, C., Lawrence, N. & Weinberger, K. Q.) Paper 1610 (Curran, 2014).
- Krizhevsky, A., Sutskever, I. & Hinton, G. E. in Advances in Neural Information Processing Systems 25 (eds Bartlett, P. et al.) 1097–1105 (Curran, 2012).
- Kiani, A. et al. Impact of a deep learning assistant on the histopathologic classification of liver cancer. npj Digit. Med.3, 23 (2020). ArticleGoogle Scholar
- Park, S.-M. et al. A mountable toilet system for personalized health monitoring via the analysis of excreta. Nat. Biomed. Eng.4, 624–635 (2020). ArticleGoogle Scholar
- Howard, J. & Ruder, S. Universal language model fine-tuning for text classification. In Proceedings of the 56th Annual Meeting of the Association for Computational Linguistics (eds Gurevych, I. & Miyao, Y.) 328–339 (Association for Computational Linguistics, 2018).
- Peters, M. E. et al. Deep contextualized word representations. In Proceedings of the 2018 Conference of the North American Chapter of the Association for Computational Linguistics: Human Language Technologies (eds Walker, M., Ji, H. & Stent, A.) 2227–2237 (Association for Computational Linguistics, 2018).
- Brown, T. et al. Language models are few-shot learners. In Advances in Neural Information Processing Systems 33 (eds. Larochelle, H., Ranzato, M., Hadsell, R., Balcan, M. F. & Lin, H.) 1877–1901 (Curran, 2020).
- Kenton, J. D. M.-W. C. & Toutanova, L. K. BERT: pre-training of deep bidirectional transformers for language understanding. in Proceedings of the 2019 Conference of the North American Chapter of the Association for Computational Linguistics: Human Language Technologies (eds Burstein, J., Doran, C. & Solorio, T.) 4171–4186 (Association for Computational Linguistics, 2019).
- Mikolov, T., Chen, K., Corrado, G. & Dean, J. Efficient estimation of word representations in vector space. Preprint at https://arxiv.org/abs/1301.3781v3 (2013).
- Pennington, J., Socher, R. & Manning, C. GloVe: global vectors for word representation. In Proceedings of the 2014 Conference on Empirical Methods in Natural Language Processing (eds Moschitti, A., Pang, B., Daelemans, W.) 1532–1543 (Association for Computational Linguistics, 2014).
- Alsentzer, E. et al. Publicly available clinical BERT embeddings. In Proceedings of the 2nd Clinical Natural Language Processing Workshop (eds Rumshisky, A., Roberts, K., Bethard, S. & Naumann, T.) 72–78 (Association for Computational Linguistics, 2019).
- Huang, K., Altosaar, J. & Ranganath, R. ClinicalBERT: modeling clinical notes and predicting hospital readmission. Preprint at https://arxiv.org/abs/1904.05342v3 (2019).
- Peng, Y., Yan, S. & Lu, Z. Transfer learning in biomedical natural language processing: an evaluation of BERT and ELMo on ten benchmarking datasets. In Proceedings of the 18th BioNLP Workshop and Shared Task (eds Demner-Fushman, D., Bretonnel Cohen, K., Ananiadou, S. & Tsujii, J.) 58–65 (Association for Computational Linguistics, 2019).
- Johnson, A. E. W. et al. MIMIC-III, a freely accessible critical care database. Sci. Data3, 160035 (2016). ArticleCASGoogle Scholar
- Sharir, O., Peleg, B. & Shoham, Y. The cost of training NLP models: a concise overview. Preprint at https://arxiv.org/abs/2004.08900v1 (2020).
- Lee, J. et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics36, 1234–1240 (2020). CASGoogle Scholar
- Beltagy, I., Lo, K. & Cohan, A. SciBERT: A pretrained language model for scientific text. In Proceedings of the 2019 Conference on Empirical Methods in Natural Language Processing and the 9th International Joint Conference on Natural Language Processing (eds Inui, K., Jiang, J., Ng, V. & Wan, X.) 3615–3620 (Association for Computational Linguistics, 2019).
- Futoma, J., Morris, J. & Lucas, J. A comparison of models for predicting early hospital readmissions. J. Biomed. Inform.56, 229–238 (2015). ArticleGoogle Scholar
- Caruana, R. et al. Intelligible models for healthcare: predicting pneumonia risk and hospital 30-day readmission. In Proc. 21st ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 1721–1730 (Association for Computing Machinery, 2015).
- Wagner, T. et al. Augmented curation of clinical notes from a massive EHR system reveals symptoms of impending COVID-19 diagnosis. Elife9, e58227 (2020). ArticleCASGoogle Scholar
- Eisman, A. S. et al. Extracting angina symptoms from clinical notes using pre-trained transformer architectures. AMIA Annu. Symp. Proc.2020, 412–421 (American Medical Informatics Association, 2020).
- Smit, A. et al. Combining automatic labelers and expert annotations for accurate radiology report labeling using BERT. In Proceedings of the 2020 Conference on Empirical Methods in Natural Language Processing (eds Webber, B., Cohn, T., He, Y. & Liu, Y.) 1500–1519 (Association for Computational Linguistics, 2020).
- Soni, S. & Roberts, K. Evaluation of dataset selection for pre-training and fine-tuning transformer language models for clinical question answering. In Proc. 12th Language Resources and Evaluation Conference 5532–5538 (European Language Resources Association, 2020).
- Sezgin, E., Huang, Y., Ramtekkar, U. & Lin, S. Readiness for voice assistants to support healthcare delivery during a health crisis and pandemic. npj Digit. Med.3, 122 (2020). ArticleGoogle Scholar
- Sakthive, V., Kesaven, M. P. V., William, J. M. & Kumar, S. K. M. Integrated platform and response system for healthcare using Alexa. Int. J. Commun. Computer Technol.7, 14–22 (2019). Google Scholar
- Comstock, J. Buoy Health, CVS MinuteClinic partner to send patients from chatbot to care. mobihealthnewshttps://www.mobihealthnews.com/content/buoy-health-cvs-minuteclinic-partner-send-patients-chatbot-care (2018).
- Razzaki, S. et al. A comparative study of artificial intelligence and human doctors for the purpose of triage and diagnosis. Preprint at https://doi.org/10.48550/arXiv.1806.10698 (2018).
- Xiong, Y., Du, B. & Yan, P. Reinforced transformer for medical image captioning. In Machine Learning in Medical Imaging (eds. Suk, H.-I., Liu, M., Yan, P. & Lian, C.) 673–680 (Springer International Publishing, 2019).
- Meng, Y., Speier, W., Ong, M. K. & Arnold, C. W. Bidirectional representation learning from transformers using multimodal electronic health record data to predict depression. IEEE J. Biomed. Health Inform.25, 3121–3129 (2021). ArticleGoogle Scholar
- Choi, E. et al. Learning the graphical structure of electronic health records with graph convolutional transformer. Proc. Conf. AAAI Artif. Intell.34, 606–613 (2020). Google Scholar
- Li, F. et al. Fine-tuning bidirectional encoder representations from transformers (BERT)–based models on large-scale electronic health record notes: an empirical study. JMIR Med. Inform.7, e14830 (2019). ArticleGoogle Scholar
- Rasmy, L., Xiang, Y., Xie, Z., Tao, C. & Zhi, D. Med-BERT: pretrained contextualized embeddings on large-scale structured electronic health records for disease prediction. npj Digital Medicine4, 86 (2021). ArticleGoogle Scholar
- Shang, J., Ma, T., Xiao, C. & Sun, J. Pre-training of graph augmented transformers for medication recommendation. in Proceedings of the Twenty-Eighth International Joint Conference on Artificial Intelligence (ed. Kraus, S.) 5953–5959 (International Joint Conferences on Artificial Intelligence Organization, 2019); https://doi.org/10.24963/ijcai.2019/825
- Li, Y. et al. BEHRT: transformer for electronic health records. Sci. Rep.10, 7155 (2020). ArticleCASGoogle Scholar
- Rao, S. et al. BEHRT-HF: an interpretable transformer-based, deep learning model for prediction of incident heart failure. Eur. Heart J.41 (Suppl. 2), ehaa946.3553 (2020). ArticleGoogle Scholar
- Qian, X. et al. Prospective assessment of breast cancer risk from multimodal multiview ultrasound images via clinically applicable deep learning. Nat. Biomed. Eng.5, 522–532 (2021). ArticleGoogle Scholar
- Xing, L., Giger, M. L. & Min, J. K. Artificial Intelligence in Medicine: Technical Basis and Clinical Applications (Academic Press, 2020).
- Reisman, M. EHRs: the challenge of making electronic data usable and interoperable. P. T.42, 572–575 (2017). Google Scholar
- Cortés, R., Bonnaire, X., Marin, O. & Sens, P. Stream processing of healthcare sensor data: studying user traces to identify challenges from a big data perspective. Procedia Comput. Sci.52, 1004–1009 (2015). ArticleGoogle Scholar
- Zhang, F., Cao, J., Khan, S. U., Li, K. & Hwang, K. A task-level adaptive MapReduce framework for real-time streaming data in healthcare applications. Future Gener. Comput. Syst.43–44, 149–160 (2015). ArticleGoogle Scholar
- El Aboudi, N. & Benhlima, L. Big data management for healthcare systems: architecture, requirements, and implementation. Adv. Bioinformatics2018, 4059018 (2018). ArticleGoogle Scholar
- Ta, V.-D., Liu, C.-M. & Nkabinde, G. W. Big data stream computing in healthcare real-time analytics. In IEEE International Conference on Cloud Computing and Big Data Analysis (ICCCBDA) 37–42 (ieeexplore.ieee.org, 2016).
- Data-Driven Healthcare Organizations Use Big Data Analytics for Big Gains White Paper (IBM Software, 2017); https://silo.tips/download/ibm-software-white-paper-data-driven-healthcare-organizations-use-big-data-analy
- Futoma, J., Simons, M., Panch, T., Doshi-Velez, F. & Celi, L. A. The myth of generalisability in clinical research and machine learning in health care. Lancet Digit. Health2, e489–e492 (2020). ArticleGoogle Scholar
- Wang, X. et al. Inconsistent performance of deep learning models on mammogram classification. J. Am. Coll. Radiol.17, 796–803 (2020). ArticleGoogle Scholar
- Nestor, B., McDermott, M. B. A. & Boag, W. Feature robustness in non-stationary health records: caveats to deployable model performance in common clinical machine learning tasks. Preprint at https://doi.org/10.48550/arXiv.1908.00690 (2019).
- Wu, E. et al. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nat. Med. https://doi.org/10.1038/s41591-021-01312-x (2021).
- Barish, M., Bolourani, S., Lau, L. F., Shah, S. & Zanos, T. P. External validation demonstrates limited clinical utility of the interpretable mortality prediction model for patients with COVID-19. Nat. Mach. Intell.3, 25–27 (2020). ArticleGoogle Scholar
- Davis, S. E., Lasko, T. A., Chen, G., Siew, E. D. & Matheny, M. E. Calibration drift in regression and machine learning models for acute kidney injury. J. Am. Med. Inform. Assoc.24, 1052–1061 (2017). ArticleGoogle Scholar
- Wang, G. et al. A deep-learning pipeline for the diagnosis and discrimination of viral, non-viral and COVID-19 pneumonia from chest X-ray images. Nat. Biomed. Eng.5, 509–521 (2021). ArticleCASGoogle Scholar
- Ning, W. et al. Open resource of clinical data from patients with pneumonia for the prediction of COVID-19 outcomes via deep learning. Nat. Biomed. Eng.4, 1197–1207 (2020). ArticleCASGoogle Scholar
- Koenecke, A. et al. Racial disparities in automated speech recognition. Proc. Natl Acad. Sci. USA117, 7684–7689 (2020). ArticleCASGoogle Scholar
- Abid, A., Farooqi, M. & Zou, J. Large language models associate muslims with violence. Nat. Mach. Intell.3, 461–463 (2021). ArticleGoogle Scholar
- Obermeyer, Z., Powers, B., Vogeli, C. & Mullainathan, S. Dissecting racial bias in an algorithm used to manage the health of populations. Science366, 447–453 (2019). ArticleCASGoogle Scholar
- Adamson, A. S. & Smith, A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 154, 1247–1248 (2018). ArticleGoogle Scholar
- Han, S. S. et al. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J. Invest. Dermatol.138, 1529–1538 (2018). ArticleCASGoogle Scholar
- Subbaswamy, A., Adams, R. & Saria, S. Evaluating model robustness and stability to dataset shift. In Proceedings of The 24th International Conference on Artificial Intelligence and Statistics (eds. Banerjee, A. & Fukumizu, K.) 2611–2619 (PMLR, 2021).
- Izzo, Z., Ying, L. & Zou, J. How to learn when data reacts to your model: performative gradient descent. In Proceedings of the 38th International Conference on Machine Learning (eds. Meila, M. & Zhang, T.) 4641–4650 (PMLR, 2021).
- Ghorbani, A., Kim, M. & Zou, J. A Distributional framework for data valuation. In Proceedings of the 37th International Conference on Machine Learning (eds. Iii, H. D. & Singh, A.) 3535–3544 (PMLR, 2020).
- Zhang, L., Deng, Z., Kawaguchi, K., Ghorbani, A. & Zou, J. How does mixup help with robustness and generalization? In International Conference on Learning Representations 2021 Paper 2273 (ICLR, 2021).
- Schulam, P. & Saria, S. Can you trust this prediction? Auditing pointwise reliability after learning. In Proceedings of the Twenty-Second International Conference on Artificial Intelligence and Statistics (eds. Chaudhuri, K. & Sugiyama, M.) 1022–1031 (PMLR, 2019).
- Liu, X. et al. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat. Med.26, 1364–1374 (2020). ArticleCASGoogle Scholar
- Cruz Rivera, S. et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Nat. Med.26, 1351–1363 (2020). ArticleCASGoogle Scholar
- Nayak, P. Understanding searches better than ever before. Google The Keywordhttps://blog.google/products/search/search-language-understanding-bert/ (2019).
- Baur, C., Albarqouni, S. & Navab, N. in OR 2.0 Context-Aware Operating Theaters, Computer Assisted Robotic Endoscopy, Clinical Image-Based Procedures, and Skin Image Analysis (eds Stoyanov, D. et al.) 260–267 (Springer International Publishing, 2018).
- Kang, E., Koo, H. J., Yang, D. H., Seo, J. B. & Ye, J. C. Cycle-consistent adversarial denoising network for multiphase coronary CT angiography. Med. Phys.46, 550–562 (2019). ArticleGoogle Scholar
- Vig, J. A multiscale visualization of attention in the transformer model. In Proceedings of the 57th Annual Meeting of the Association for Computational Linguistics: System Demonstrations (Costa-jussà, M. R. & Alfonseca, E.) 37–42 (Association for Computational Linguistics, 2019).
Acknowledgements
This work was supported in part by the National Institutes of Health via grants F30HL156478 (to A.Z.), R01CA227713 (to L.X.), R01CA256890 (to L.X.), P30AG059307 (to J.Z.), U01MH098953 (to J.Z.), P01HL141084 (to J.C.W), R01HL163680 (to J.C.W), R01HL130020 (to J.C.W), R01HL146690 (to J.C.W.) and R01HL126527 (to J.C.W.); by the National Science Foundation grant CAREER1942926 (to J.Z.); and by the American Heart Association grant 17MERIT3361009 (to J.C.W.). Figures were created with BioRender.com.
Author information
Authors and Affiliations
- Stanford Cardiovascular Institute, School of Medicine, Stanford University, Stanford, CA, USA Angela Zhang & Joseph C. Wu
- Department of Genetics, School of Medicine, Stanford University, Stanford, CA, USA Angela Zhang
- Greenstone Biosciences, Palo Alto, CA, USA Angela Zhang & Joseph C. Wu
- Department of Computer Science, Stanford University, Stanford, CA, USA Angela Zhang & James Zou
- Department of Radiation Oncology, School of Medicine, Stanford University, Stanford, CA, USA Lei Xing
- Department of Biomedical Informatics, School of Medicine, Stanford University, Stanford, CA, USA James Zou
- Departments of Medicine, Division of Cardiovascular Medicine Stanford University, Stanford, CA, USA Joseph C. Wu
- Department of Radiology, School of Medicine, Stanford University, Stanford, CA, USA Joseph C. Wu
- Angela Zhang








